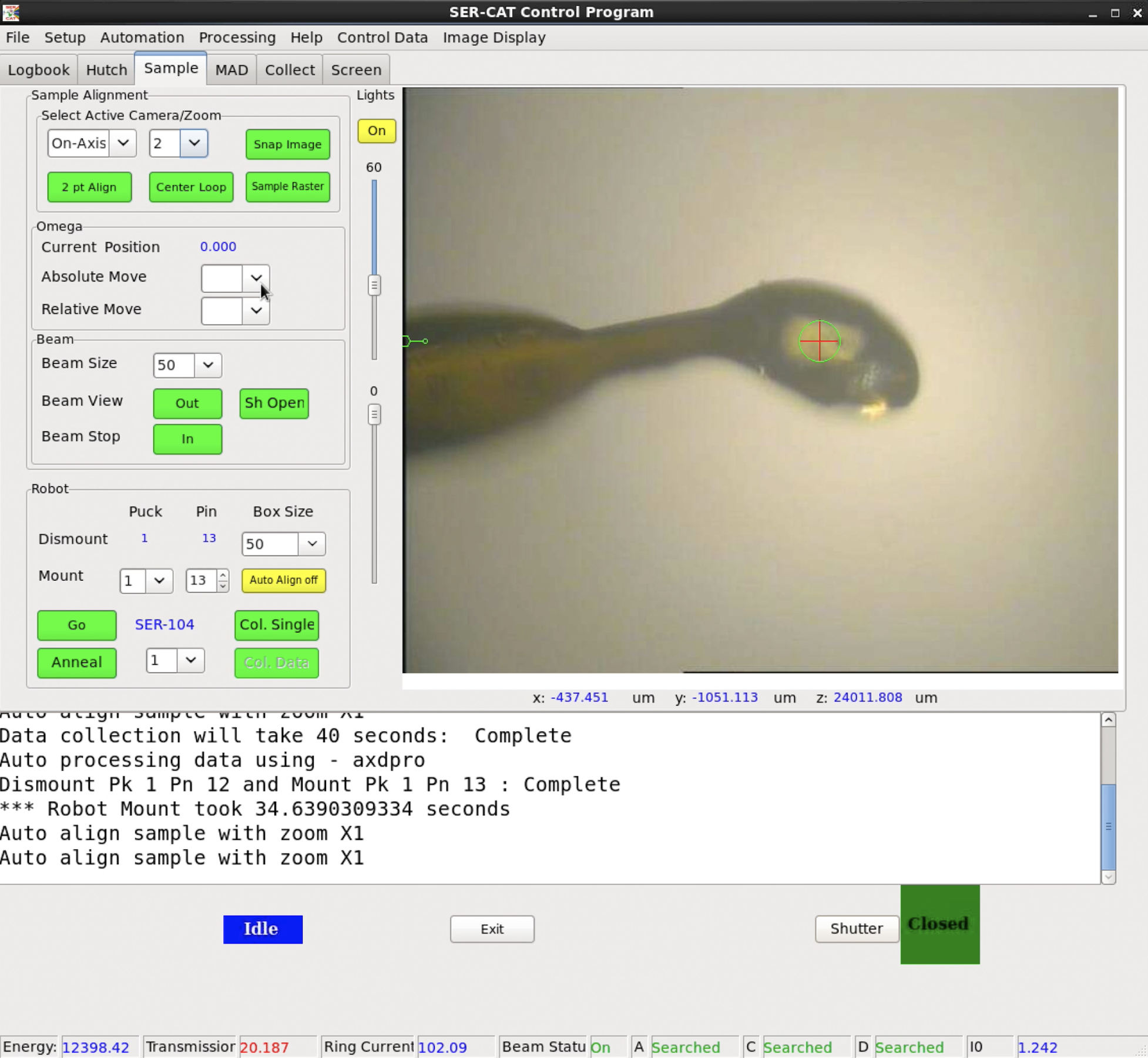
SERGUI interface, at SER-CAT Beamlines
The SouthEast Regional Collaborative Access Team (SER‐CAT) is an organization consisting of 19 Member and 4 Associate User institutions, most of which are located in the SouthEastern region of the USA.
SER‐CAT's beamlines are located at Sector 22, Advanced Photon Source (APS), Argonne National Laboratory (ANL).
As a part of the APS Upgrade, they are presently being converted to two state‐of‐the‐art Insertion Device beamlines (22‐ID‐D, variable wavelength; and the new 22‐ID‐E, fixed wavelength).
Future emphasis will be placed on de novo SAD/MAD structure determination, high‐resolution structural analyses, micro crystals, large unit cells, drug design, soft X‐ray data collection, full hands‐free automated data collection, and serial synchrotron crystallography.
SER‐CAT is operated by the University of Georgia; Dr. John P. Rose, Director.
AWARDS
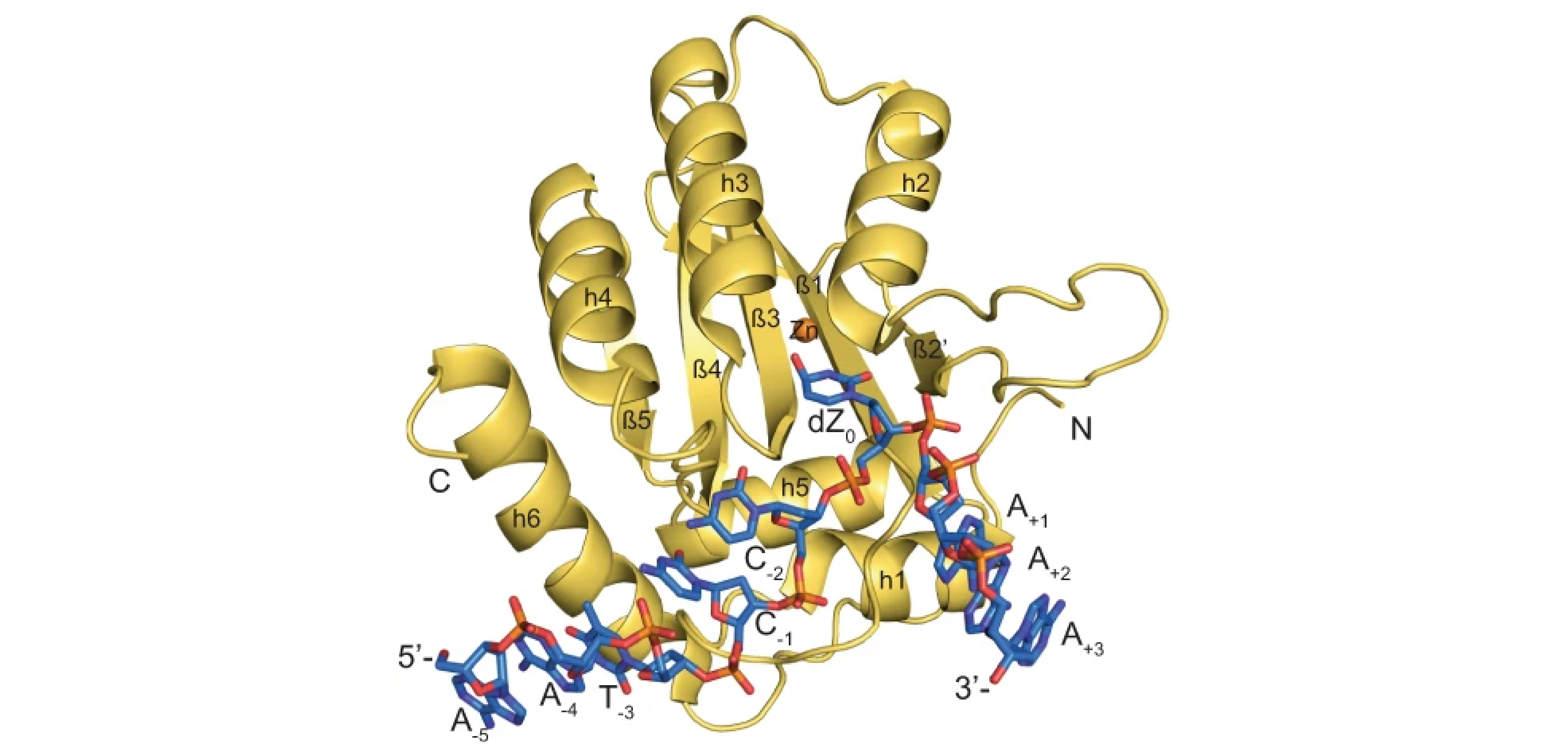
Structure of the catalytically active APO-BEC3G bound to a DNA oligonucleotide inhibitor reveals tetrahedral geometry of the transition state
The 2023 SER-CAT Outstanding Science Award was presented to Dr. Hiroshi Matsuo, of the Center for Cancer Research, National Cancer Institute, Frederick, MD, USA, for his outstanding work entitled: Structure of the catalytically active APO-BEC3G bound to a DNA oligonucleotide inhibitor reveals tetrahedral geometry of the transition state.
Award was presented by: SER-CAT Director Dr. John P. Rose.
- Authors: Atanu Maiti, Adam K. Hedger, Wazo Myint, Vanivilasini Balachandran, Jonathan K. Watts, Celia A. Schiffer, Hiroshi Matsuo.
- Nat Commun. 2022 Nov 19;13(1):7117. doi: 10.1038/s41467-022-34752-1.
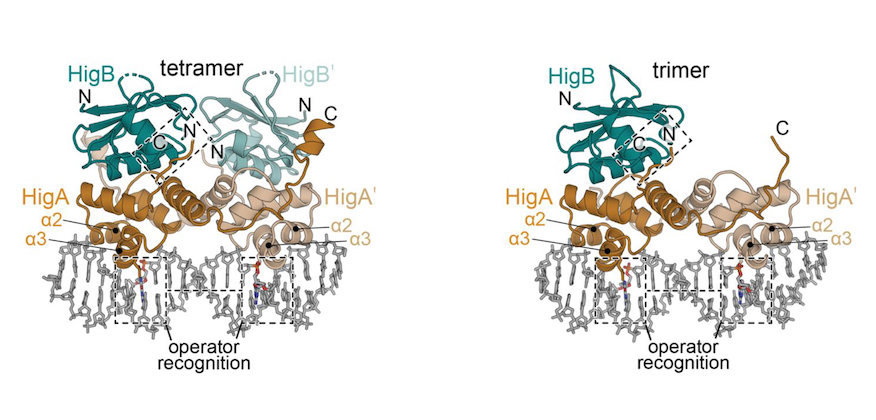
Molecular mechanism regulating transcriptional control of the hig toxin‐antitoxin locus of antibiotic-resistance plasmid Rts1 from Proteus vulgaris.
The 2022 SER-CAT Young Investigator Award was presented to Dr. Ian Pavelich, of Emory University School of Medicine, Atlanta, GA, USA, for his outstanding work entitled: Molecular mechanism regulating transcriptional control of the hig toxin-antitoxin locus of antibiotic-resistance plasmid Rts1 from Proteus vulgaris.
Award was presented by: SER-CAT Director Professor Bi-Cheng Wang.
- Authors: Ian Pavelich, Marc A. Schureck, Dongxue Wang, Eric D. Hoffer, Michelle Boamah, Nina Onuoha, Stacey J. Miles, C. Denise Okafor, Christine M Dunham.
- https://www.biorxiv.org/ content/ 10.1101/ 2021.03.04.434028v1.full.
SCIENCE HIGHLIGHTS
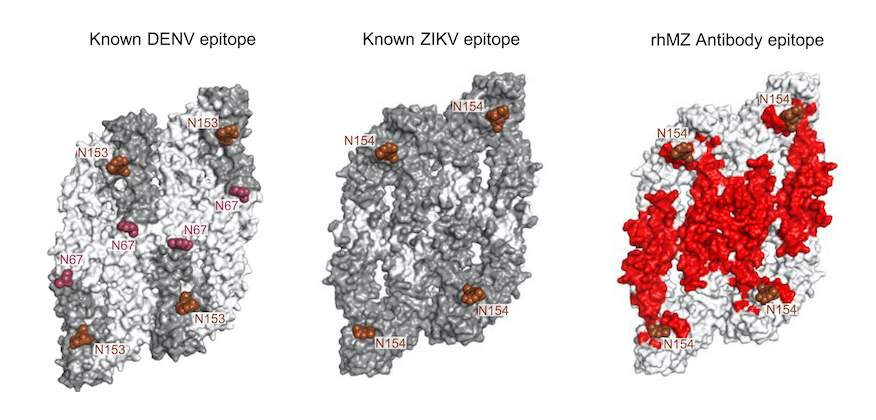
Zika-specific neutralizing antibodies targeting inter-dimer envelope epitopes.
Zika virus (ZIKV) is an emerging pathogen that causes devastating congenital defects. The overlapping epidemiology and immunologic cross-reactivity between ZIKV and dengue virus (DENV) pose complex challenges to vaccine design, given the potential for antibody-dependent enhancement of disease. Therefore, classification of ZIKV-specific antibody targets is of notable value...
- Authors: Rajeshwer S Sankhala, Vincent Dussupt, Gina Donofrio, Gregory D Gromowski, Rafael A De La Barrera, Rafael A Larocca, Letzibeth Mendez-Rivera, Anna Lee, Misook Choe, Weam Zaky, Grace Mantus, Jaime L Jensen, Wei-Hung Chen, Neelakshi Gohain, Hongjun Bai, Michael K McCracken, Rosemarie D Mason, David Leggat, Bonnie M Slike, Ursula Tran, Ningbo Jian, Peter Abbink, Rebecca Peterson, Eric Araujo Mendes, Rafael Freitas de Oliveira Franca, Guilherme Amaral Calvet, Ana Maria Bispo de Filippis, Adrian McDermott, Mario Roederer, Mayda Hernandez, Amie Albertus, Edgar Davidson, Benjamin J Doranz, Morgane Rolland, Merlin L Robb, Rebecca M Lynch, Dan H Barouch, Richard G Jarman, Stephen J Thomas, Kayvon Modjarrad, Nelson L Michael, Shelly J Krebs, M Gordon Joyce.
- Cell Rep. 2023 Aug 29;42(8):112942. doi: 10.1016/j.celrep.2023.112942. Epub 2023 Aug 9.
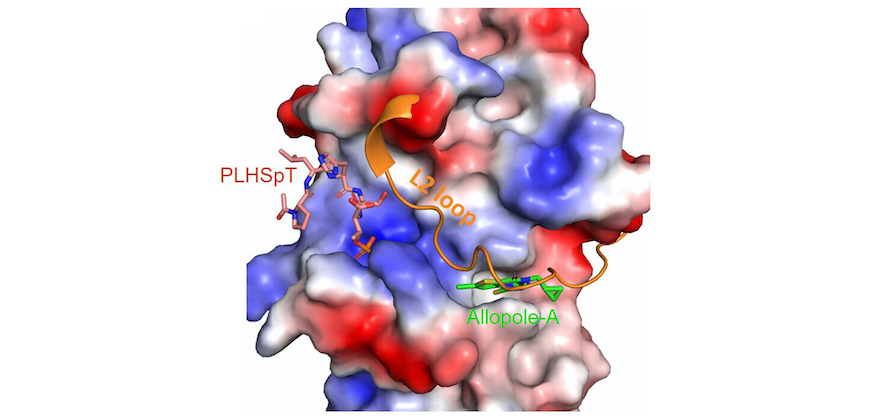
Specific inhibition of an anticancer target, polo-like kinase 1, by allosterically dismantling its mechanism of substrate recognition.
Polo-like kinase 1 (Plk1) is considered an attractive target for anticancer therapy. Over the years, studies on the noncatalytic polo-box domain (PBD) of Plk1 have raised the expectation of generating highly specific protein-protein interaction inhibitors. However, the molecular nature of the canonical PBD-dependent interaction, which requires extensive water network-mediated interactions with its phospholigands, has hampered efforts to identify small molecules suitable for Plk1 PBD drug discovery. Here, we report the identification of the first allosteric inhibitor of Plk1 PBD, called Allopole...
- Authors: Jung-Eun Park, Klara Kirsch, Hobin Lee, Paola Oliva, Jong Il Ahn, Harsha Ravishankar, Yan Zeng, Stephen D Fox, Samuel A Kirby, Pooja Badhwar, Thorkell Andresson, Kenneth A Jacobson, Kenneth A Jacobson, Kyung S Lee.
- Proc Natl Acad Sci U S A. 2023 Aug 29;120(35):e2305037120. doi: 10.1073/pnas.2305037120.
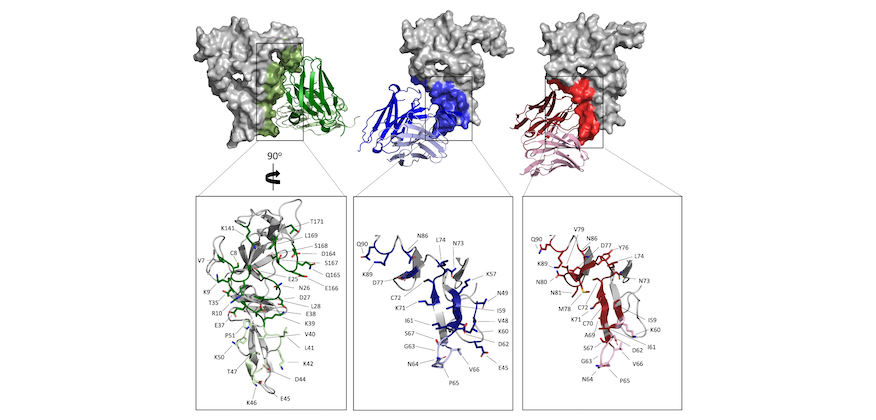
A human antibody epitope map of the malaria vaccine antigen Pfs25.
Pfs25 is a leading antigen for a malaria transmission-blocking vaccine and shows moderate transmission-blocking activity and induction of rapidly decreasing antibody titers in clinical trials. A comprehensive definition of all transmission-reducing epitopes of Pfs25 will inform structure-guided design to enhance Pfs25-based vaccines, leading to potent transmission-blocking activity...
- Authors: Niharika Shukla, Wai Kwan Tang, Camila H Coelho, Carole A Long, Sara A Healy, Issaka Sagara, Kazutoyo Miura, Patrick E Duffy, Niraj H Tolia.
- NPJ Vaccines. 2023 Aug 4;8(1):108. doi: 10.1038/s41541-023-00712-z.
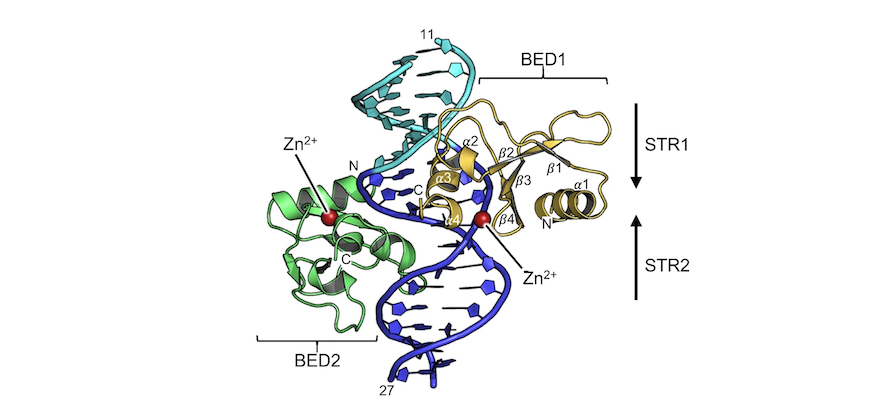
Zinc-finger BED domains drive the formation of the active Hermes transpososome by asymmetric DNA binding.
The Hermes DNA transposon is a member of the eukaryotic hAT superfamily, and its transposase forms a ring-shaped tetramer of dimers. Our investigation, combining biochemical, crystallography and cryo-electron microscopy, and in-cell assays, shows that the full-length Hermes octamer extensively interacts with its transposon left-end through multiple BED domains of three Hermes protomers...
- Authors: Laurie Lannes, Christopher M Furman, Alison B Hickman, Fred Dyda.
- Nat Commun. 2023 Jul 25;14(1):4470. doi: 10.1038/s41467-023-40210-3.
NEWS
20th Annual SER‐CAT Symposium; 2023
The 20th Annual SER‐CAT Symposium was held on November 2-3, 2023; it was hosted by the University of Alabama, at Huntsville AL.
The UAH ‐ SER‐CAT Structural Biology Symposium website remains live; full agenda is available on website.
Previous Symposia:
A table is also available, listing:
- Previous Symposia Dates
- Institutional Hosts
- Local Organizers

BEAMLINE INFORMATION

Looking for Beamtime?
Members, Associate Users, and GUP-Users are scheduled for beamtime using these guidelines.
Looking to become a GUP User at SER-CAT?

Award Nominations
Information for nominating Outstanding Science or Young Investigator for SER-CAT Annual Awards.